The mission of the PLN Foundation is crystal clear: to find a safe, affordable and effective treatment for PLN R14del. A new development may offer hope: the experimental drug I-1c, a gene therapy currently being tested in several heart patients, could also be effective in the heart muscle disease PLN. A small number of PLN patients now have the opportunity to participate in a clinical trial with I-1c and thus contribute to research to determine whether this potential treatment is effective in PLN. Although I-1c has not yet been proven as an effective treatment, initial results are positive. In this article, we explain more about what I-1c is, what results have been obtained so far, and the current status of testing in PLN-.
Current treatments for PLN are inadequate
The PLN mutation PLN R14del causes two problems in the heart: arrhythmias and dilatation (dilation of the heart) resulting in reduced pump function (heart failure). These problems also occur in heart patients without the PLN mutation. Therefore, PLN patients, like other heart patients, now receive general treatments such as beta-blockers. Because these treatments are not curative, we do a lot of research into treatments that do cure or prevent PLN. To really get to the root of the problem, the best option is to fix the bug in the DNA or capture the product that is being written off the gene. However, these options will take several years to reach the clinical trial stage. Another option is a more general treatment that intervenes close to the cause of PLN R14del. In this article, we discuss I-1c, a gene therapy that could be such a general treatment.
The mechanism behind I-1c
I-1c stands for inhibitor-1 which is constitutively (meaning always) active. I-1c is involved in calcium metabolism (Figure 1). Cardiac muscle cell contraction is regulated by calcium. Calcium is released from the cell’s internal storage, induces contraction and is then pumped back into storage by SERCA. The amount of calcium in the storage is important because if there is not enough calcium in the storage, the heart cannot contract vigorously, and if the storage is overfilled, spontaneous contractions (an incipient arrhythmia) may occur. SERCA is therefore strictly regulated. PLN is one of the proteins that inhibits SERCA. When the heart needs to beat faster, I-1c inhibits PLN and therefore SERCA can become more active.
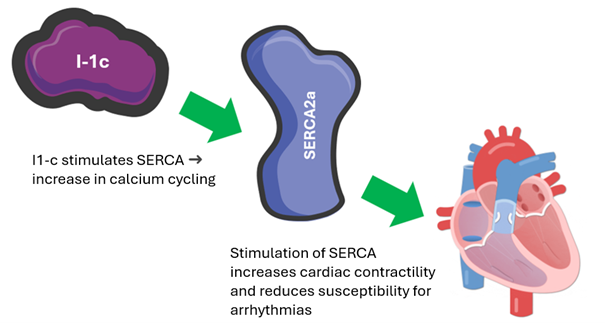
Figure 1 Operation of I-1c
I-1c is administered as a gene, but you cannot administer a gene separately as a pill or injection. Therefore, it is packaged in a jacket, a virus. This is an efficient and relatively safe way. The disadvantage of this virus is that it must be administered via an injection into the heart. This procedure is similar to cardiac catheterization. The advantage, however, is that the gene stays in the heart. Therefore, you do not have to take a pill every day; a one-time treatment is sufficient.
Phase 1 clinical trial
Following thorough evidence that I-1c is safe and effective in cultured heart muscle cells and animals, the first study in humans has taken place. The phase 1 study of I-1c looked at the safety of the drug and gathered initial insights about possible effects on heart function in patients with heart problems. Patients in this study were administered a low or high dose of the drug and then followed for a year. Importantly, the phase 1 study showed that the drug was well tolerated by the body. Indeed, no serious immune responses or significant liver damage were observed.
Additionally, the results show some positive changes, such as improvements in heart failure classification (NYHA classification), heart pump function (LVEF), and endurance (6MWT). However, caution is needed because in a phase 1 study you cannot tell if a drug is indeed effective. To be truly sure of the efficacy of I-1c, follow-up studies are needed with a larger group of participants and a placebo control.
Prove that I-1c also works for PLN
PLN Foundation is in good contact with AskBio, the company developing the drug I-1c. As a result, I-1c could also be tested on models made specifically for the PLN mutation. Because the first study of I-1c was only conducted in people with heart failure (without PLN mutation), it is important to see if the drug also works for PLN patients. To do this, we are using models of the disease, such as “spheres” of cultured heart muscle cells (spheroids) from stem cells of PLN carriers and mice with the PLN mutation.
In the first part of the study, I-1c was administered to cultured heart muscle cells via a virus. This caused improvements in how the cells process calcium and how forcefully the cells contract, which is important for a properly functioning heart. In addition, a decrease was observed in connective tissue proteins that are often elevated in heart disease.
The second part of the study looked at whether I-1c could reduce cardiac arrhythmias in mice with the PLN mutation. Normally, these mice almost always develop cardiac arrhythmias when stimulated, for example by administration of caffeine. But in mice previously treated with I-1c, these arrhythmias were much less common. Although these are good indications, it has not yet been tested whether I-1c can also counteract heart enlargement in PLN patients. In animal studies of other heart diseases, I-1c was found to do this.
These results provide evidence that I-1c may also have benefits for PLN carriers. However, more research is needed to be sure that it is safe and effective for PLN patients.
Clinical studies with PLN patients
There is good news for PLN carriers: an additional phase 1 study has begun in the United States specifically for people with the PLN mutation. This new study arm is investigating the safety and potential effects of the drug I-1c specifically in PLN patients.
In addition, a phase 2 trial was recently launched in both the United States and Europe to look at the efficacy of I-1c. PLN patients will also be included in this trial. In the Netherlands, Amsterdam UMC and UMC Utrecht are participating. Only patients who meet specific medical criteria are eligible for this study. In this study, not all participants will receive the drug. To properly compare whether I-1c really makes a difference, there are two groups receiving I-1c (in high and low doses) and one group receiving a placebo (similar administration but without the active ingredient).
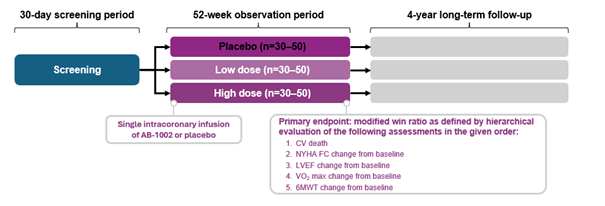
Study design phase 2 clinical trial presented by AskBio at the 2023 AHA Congress
More information about the European clinical trial can be found here: https://clinicaltrials.gov/study/NCT05598333
Conclusion
This article shows that I-1c may offer a potential treatment for PLN patients. Although the efficacy of I-1c has not yet been definitively proven, initial results are positive. If you are considering participating in this study, we recommend that you contact your cardiologist. For now, I-1c is the only possible treatment where PLN carriers are specifically a part of the clinical trial. With this, I-1c opens a new door to a possible treatment for PLN.

