How are cardiac arrhythmias actually caused? We know that cardiac arrhythmias occur in PLN and that PLN regulates calcium. Calcium is important for the conversion of an electrical signal into the contraction of the heart. Thus, it is plausible that the two are related. In this article, we discuss a study that looked very closely at how a disturbance of calcium might lead to arrhythmias (arrhythmia).
For this study, mouse hearts were used that we wrote about previously. The previous article showed that in mouse PLN hearts there are no changes in structure, but changes are found in electrical activation in the heart. These changes can be seen at the molecular level as well as on, for example, a heart movie. This article builds on these findings by examining whether altered electrical activation leads to cardiac arrhythmias.
THE HUMAN HEART AND THE MOUSE HEART
Studying cardiac arrhythmias in mice is not so easy. After all, the mouse heart beats about 600 times per minute! Our human heart, on the other hand, contracts about 70 times per minute at rest. Interestingly, however, our heart rate on exercise increases to 120-150 beats per minute (a 70-110% increase), while a mouse heart does not exceed 700-800 times (a 17-33% increase). Thus, the human heart is much more adaptable. Because of their high heart rate, mice are less likely to suffer from cardiac arrhythmias: before the slightest deviation occurs, a new heartbeat comes along and straightens everything out.
By themselves, therefore, PLN hearts do not exhibit any disturbance of heart rhythm. After stimulation with a substance similar to adrenaline, this changes. PLN hearts are more likely to have arrhythmias than healthy hearts. When we zoom in, the right ventricle in particular appears to be abnormal. The electrical activation is slower there, there are more differences in the electrical activation and the heart cells do not respond well to adrenaline.
Electrical activity
In this next step, advanced imaging is used to see how electrical activity moves through the heart. What emerges? In the PLN heart, a kind of traffic jam forms (Figure 1). The electrical signal is transmitted slowly while a new signal is already arriving in the meantime. Added to this is a second problem: PLN hearts contain areas of fat and connective tissue.
These spots are badly activated by the electrical signals, slowing down or even blocking the signal. Something can still happen at these spots. Normally the signal is transmitted in a straight line. But because there is now a spot where the signal is badly transmitted, a spiral, a kind of whirlwind, is created around this spot.
Vertebrae find in the heart
This creates an island of cells that are not activated. This disruption can be the beginning of an arrhythmia. In healthy mouse hearts, such whirlwinds also sometimes occur, but in PLN hearts they have some characteristics that make them more likely to become a problem. In PLN hearts, the whirlwind can occur at a lower heart rate, making them less easily repaired. Because of the problems in PLN hearts, whirlwinds occur more often around regions of delayed activation and in the right ventricle.
In addition, whirlwinds last longer and spin faster than the heartbeat at which they originate. This is related: there are problems with conduction in the right ventricle, and because the vortex spins faster than the heartbeat, it is more difficult to stop it. Finally, the vortices move faster in PLN hearts and there are abnormalities in the tissue that go beyond simply an increased risk of arrhythmias.
This complex study reveals part of the mechanism behind cardiac arrhythmias in PLN. This shows that there are changes in cardiac tissue that can lead to arrhythmias before there are changes in the structure or function of the heart. These changes can block the electrical signal or cause an eddy that disrupts the signal. In doing so, this study unravels a piece of the mechanism behind cardiac arrhythmias in PLN.
THE AUTHORS OF THIS STUDY
This research was led by Francesca Stilltano and Fadi Akar. Professor Akar is famous for his research on artimia. Akar worked in New York for many years and is now affiliated with the famous Yale University. Francesca Stillitano has worked in New York for many years and has been conducting PLN research almost full-time from Utrecht since 2022. First author is Nour Raad. She works in Germany as a cardiologist, but has previously done research on PLN in New York.
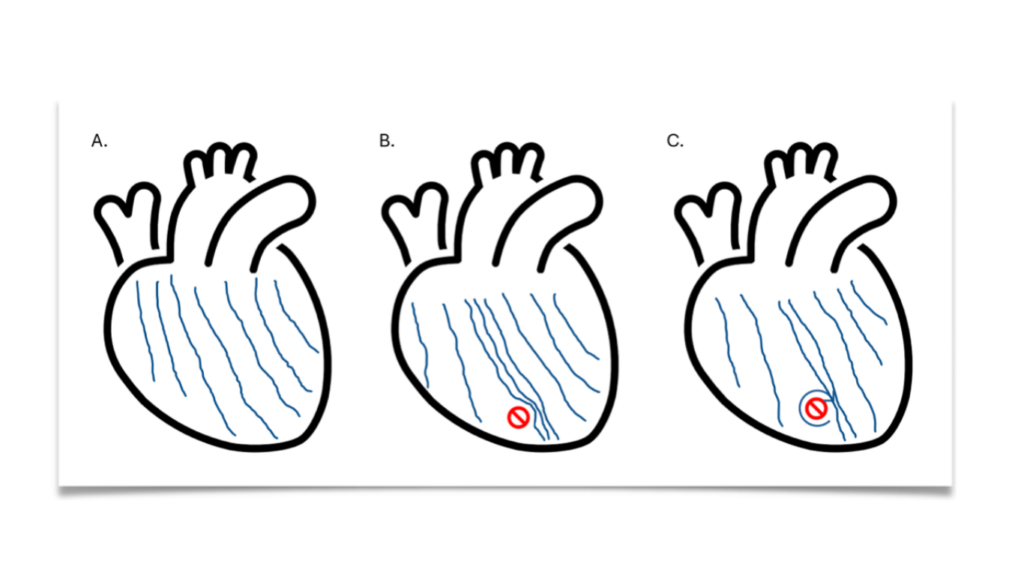
Figure 1 Drawing of the waves of electrical activation. In a healthy heart (A), the electrical waves move fairly straight and with equal spacing. In a PLN heart (B), the waves are slowed down at the separation of the left and right halves of the heart. This creates a traffic jam. In addition, there is fat and connective tissue present (yellow spot) where the signal is not properly transmitted. An eddy can form around this spot (C).
Council, 2021, Circulation Arrhythmia Mechanism and Dynamics in a Humanized Mouse Model of Inherited Cardiomyopathy due to Phospholamban R14del Mutation
