With GIP (gene therapy information packet), we take you into the world of gene therapy. In recent years there has been a lot of research into gene therapy. This has provided insight into whether and how gene therapy can help with a treatment for PLN-R14del. We will take you into the world of gene therapy. In this fourth lesson, we look at where we are now regarding gene therapy.
Overview gene therapy information packet:
- The gene therapy information packet!
- BIP – Lesson 1: Cells and DNA.
- BIP – Lesson 2: Possible gene therapies.
- BIP – Lesson 3: Risks and concerns.
- GIP – Lesson 4: Existing gene therapies.
In three lessons, we have dwelt on what gene therapy is, what options there are for PLN and what disadvantages of gene therapy are. Now we will look at different gene therapies already on the market, research on gene therapy for heart disease and the research in PLN.
Gene therapies on the market – some examples
The first gene therapy approved in Europe in 2012 was glybera. Glybera was a drug for patients who have too high fat levels due to a mutation, causing inflammation of the pancreas. Was, because of the high price and extremely low number of patients, the drug is no longer on the market. A success, but also a flop.
The first gene therapy authorized in America (in 2016) is luxturna. Luxturna is a therapy for an eye disease that results in blindness. As a result of the therapy, patients regain most of their vision. Although long-term effects remain to be seen, the results after 3 and 5 years are positive. Another therapy is Spinraza. Spinraza is aimed at restoring muscle function in patients with muscle disease. Here the effect is more limited and ongoing treatment is needed. The cost of this is €375,000 per year.
Gene therapy for heart disease
Have there been previous clinical trials with gene therapy for heart disease in general? Yes, there have been a number of small and large studies. Here we dwell on I-1c and SERCA, two therapies that have (come) far.
SERCA was one of the first gene therapies for a non-genetic disease to be studied in humans. SERCA is the protein regulated by PLN. Several years ago, a phase 2 clinical trial found that treatment with SERCA did not improve heart failure. There are several explanations for this. The dose was probably too low and not enough heart cells were reached. However, it is also possible that SERCA itself is not a good strategy for improving the disease. Regardless, important lessons were learned that are now being applied. For example, a different route of administration, a different AAV and a higher dose are now being used for I-1c.
Research on I-1c has already been mentioned (Figure 1). I-1c is a protein that regulates PLN, among other things. I-1c has passed its first clinical phase, which is primarily designed to investigate safety. In this phase, the treatment proved safe and even slight improvement was seen in treated patients. The next phase will see if I-1c does indeed provide improvement. This phase begins in 2025 and compares untreated patients with two groups of patients, one treated with a low dose and one with a high dose. What’s special is that PLN patients can participate in this study. In an extension of the phase 1 trial, American patients can participate. In addition, PLN patients in the Netherlands can participate in the phase 2 trial. The PLN Foundation is in close contact with Asklepios BioPharmaceuticals, the company developing I-1c.
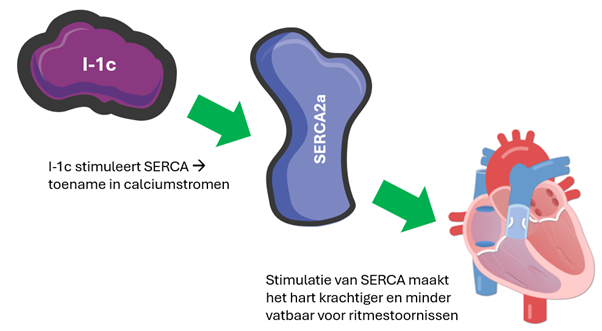
Figure 1 Operation of I-1c
Gene therapy for PLN
Some examples of gene therapies being tested for PLN have already been mentioned. It is not easy to bring such a treatment to market at an affordable price. To facilitate this process, the PLN foundation owns PLaN therapeutics. Through this company, the foundation collaborates with several universities and companies. Currently, a number of possible therapies including prime editing are being tested at different universities and companies. With prime editing it is possible to repair the error in the DNA, i.e. the cleanest treatment. This research is taking place in Leiden, Utrecht, Munich and the prestigious Stanford University in America, among others.
In addition to prime editing, research is also being conducted on CRIPSR/Cas9. A scientific paper on CRISPR-Cas9 in PLN-R14del mice was published in 2022. These mice have an enlarged heart and a higher risk of cardiac arrhythmias after stimulation. This corresponds to the two phenotypes of PLN patients. The study compared healthy and diseased mice treated with CRISPR/Cas9 in an AAV. This therapy had a positive effect: sensitivity to arrhythmias decreased and the heart did not enlarge. The heart of the diseased mice returned to near full health after treatment.
When it was then looked at how many of the heart cells were now actually corrected, it turned out to be only 10%. This shows two things: first, the efficiency to correct the mutation is very low, which is the bad news. The good news is that despite that low efficiency, the therapy was effective. Apparently, by no means all cells need to be healthy to ensure that there is still a substantial improvement. A similar therapy is currently being developed by Tenaya Therapeutics, an American company actively conducting PLN research. This treatment involves destroying the altered PLN gene so that the altered PLN can no longer disrupt the cell.
So several options for treatments are being explored. Which treatment works best and can be most easily brought to the patient needs further research. It is promising that there is so much research being done in the academy and by many different pharmaceutical companies on treatments for PLN. To complete the GIP (gene therapy information packet), we would like to look at your questions. Do you have a question about gene therapy or a treatment for PLN? If so, please email info@plnheart.org with your question.